What is a Water Softener system ?
The ion exchange water softener is one of the most common tools of water treatment. Its function is to remove scale-forming calcium and magnesium ions from hard water. In many cases soluble iron (ferrous) can also be removed with softeners. A standard water softener has four major components: a resin tank, resin, a brine tank and a valve or controller.
The softener resin tank contains the treated ion exchange resin - small beads of polystyrene. The resin beads initially adsorb sodium ions during regeneration. The resin has a greater affinity for multi-valent ions such as calcium and magnesium than it does for sodium. Thus, when the hard water containing the Calcium and Magnesium Ions is passed through the resin bed, the calcium and magnesium ions adhere to the resin, releasing the sodium ions until equilibrium is reached. The water softener has exchanged its sodium ions for the calcium and magnesium ions in the water.
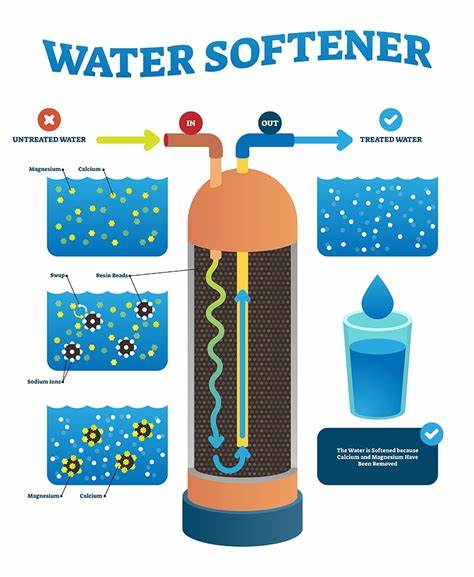
What does a Water Softener do?
Water softeners use a process called ion exchange. The softener unit contains a column filled with beads of a resin which remove the dissolved calcium and magnesium ions from the hard water flowing through it, replacing them with sodium. Periodically, (typically once a day) the softener regenerates the resin by washing with brine (sodium chloride solution), carrying the calcium and magnesium to drain, and leaving fresh sodium ions on the resin ready for the next treatment cycle. Regeneration is usually triggered automatically by either an electronic timer or mechanical water flow meter. The unit has to be refilled with salt (in the form of granules, small tablets or blocks) typically once a month.
Water suppliers define the level of water hardness (total hardness) in mg/l (milligrams per litre) of calcium carbonate. The water supply is considered to be hard if it is above 200 mg/l. 60% of water supplies in the US are hard and are typically 300 to 350 mg/l total hardness. Softening generally reduces the hardness to less than 10 mg/l.
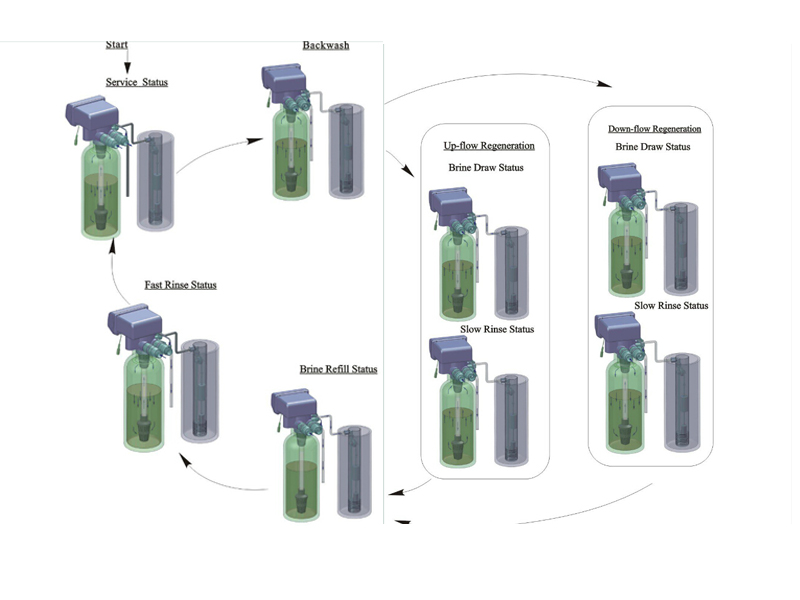
How does water softener work?(Principles of Ion Exchange to Soften Water )
Calcium and magnesium ions are atoms having a positive electrical charge, as do sodium and potassium ions. Ions of the same charge can be exchanged. In the ion exchange process, a granular substance (usually a resin) that is coated with sodium or potassium ions comes into contact with water containing calcium and magnesium ions. Two positively charged sodium or potassium ions are exchanged (released into the water) for every calcium or magnesium ion that is held by the resin. This “exchange or trade” happens because sodium or potassium are loosely held by the resin. In this way, calcium and magnesium ions responsible for hardness are removed from the water, held by the resin, and replaced by sodium or potassium ions in the water. This process makes water “soft.” Eventually, a point is reached when very few sodium or potassium ions remain on the resin, thus no more calcium or magnesium ions can be removed from the incoming water. The resin at this point is said to be “exhausted” or “spent,” and must be “recharged” or “regenerated.”



Project:

